"Time Travelling Microscope" Unbelievable Dental Anesthetics in Octocaine Part 3
WHY is Shapeshifting NT in Dental Anesthetics?
Nano Technology in Dental Anesthetics
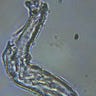
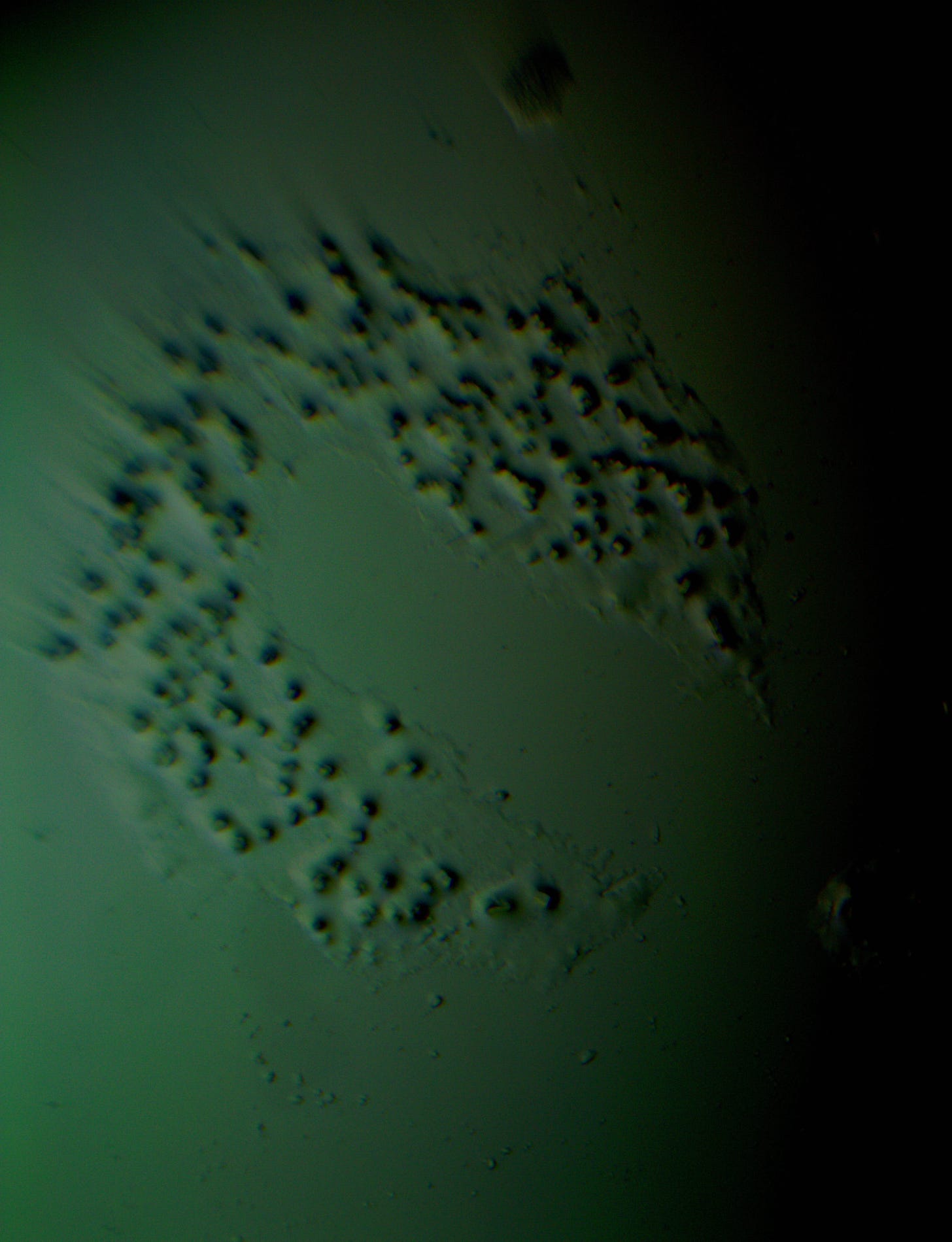
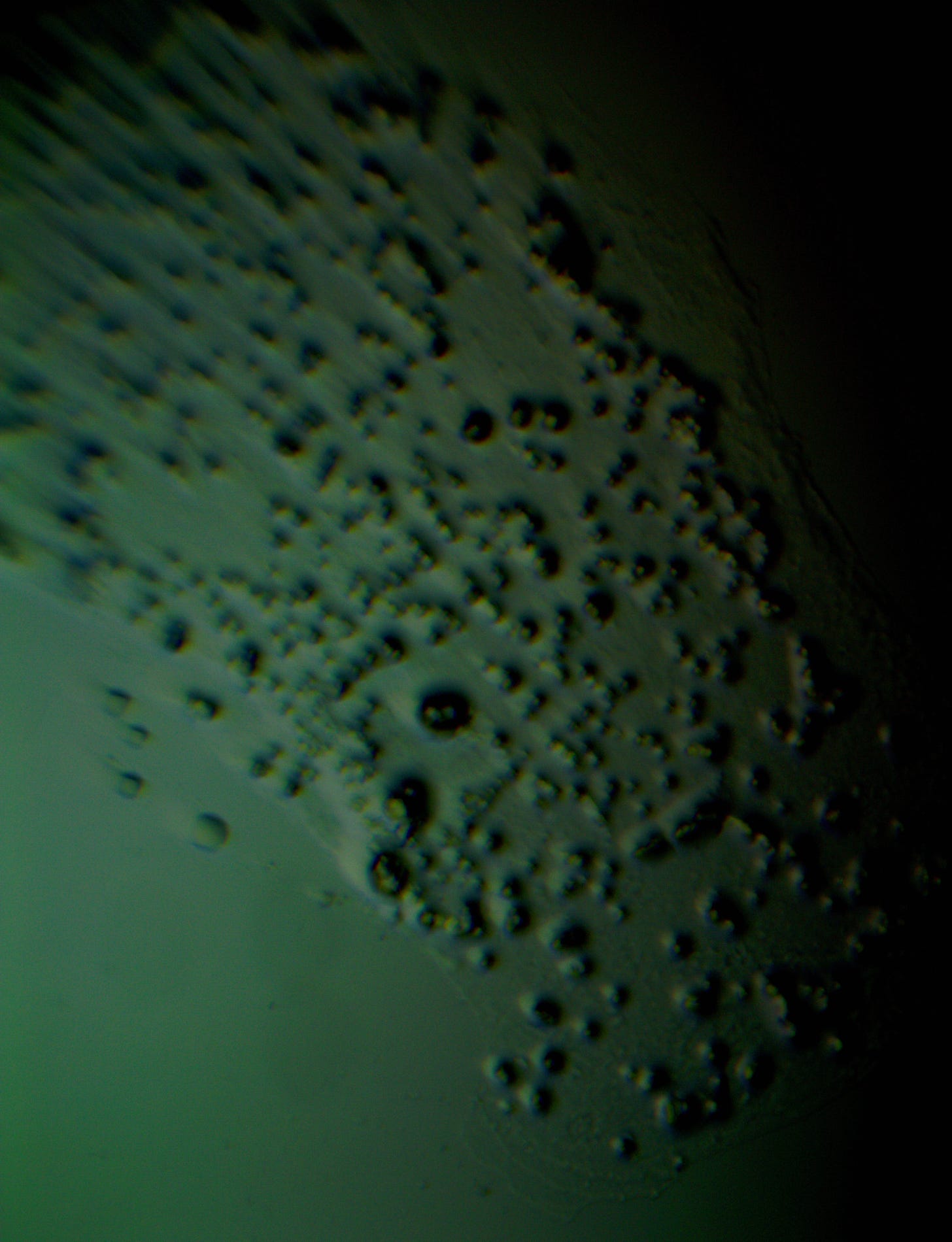
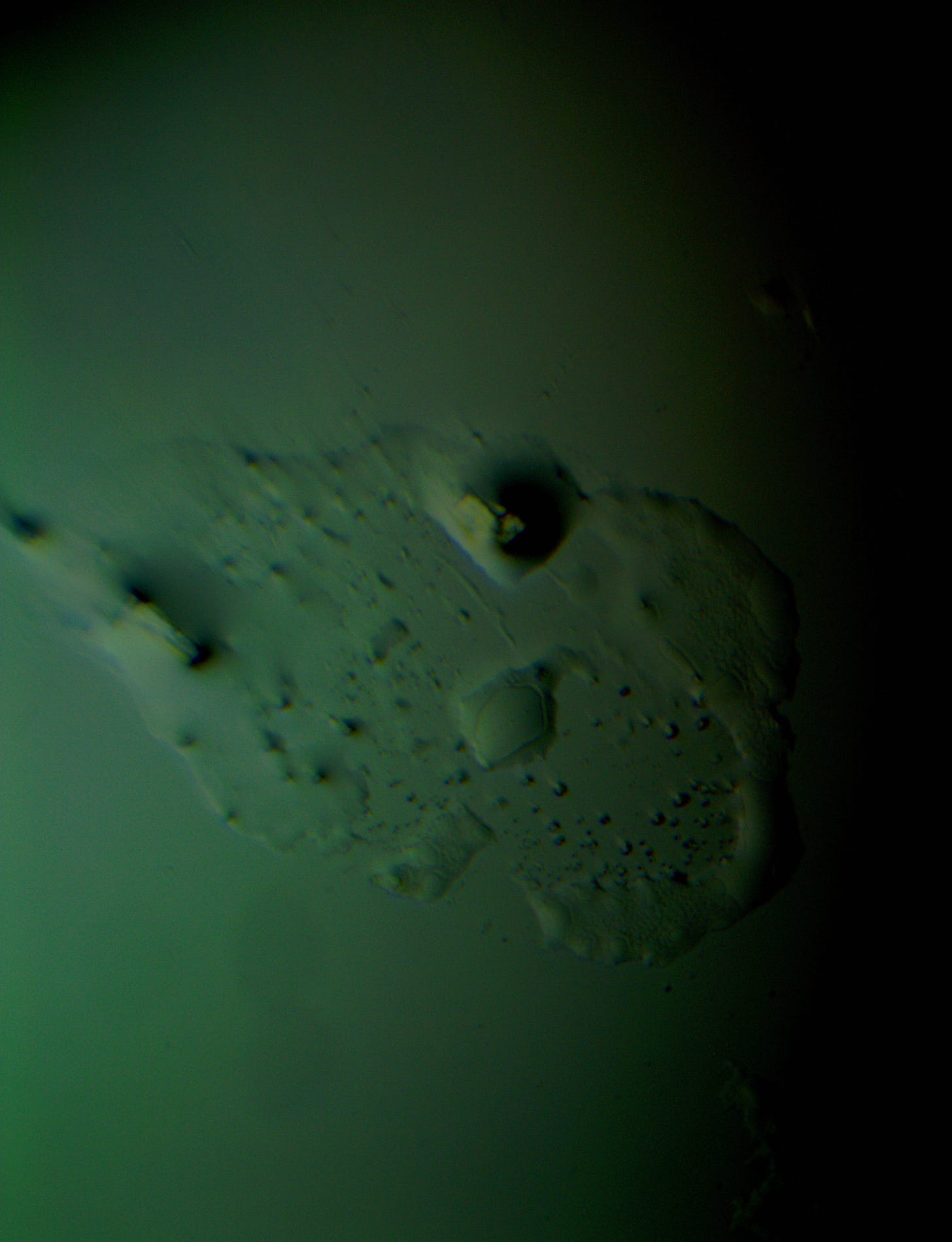
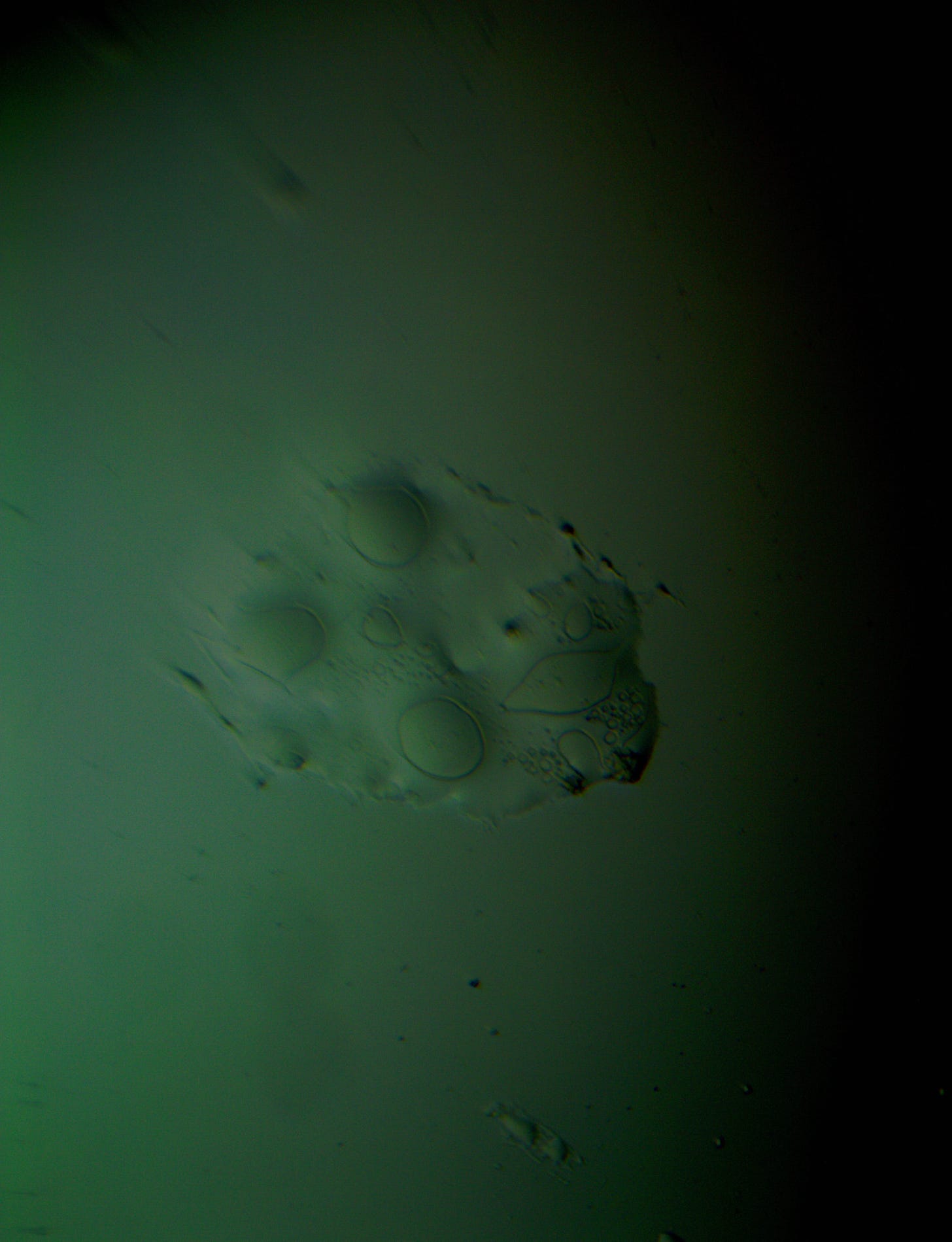
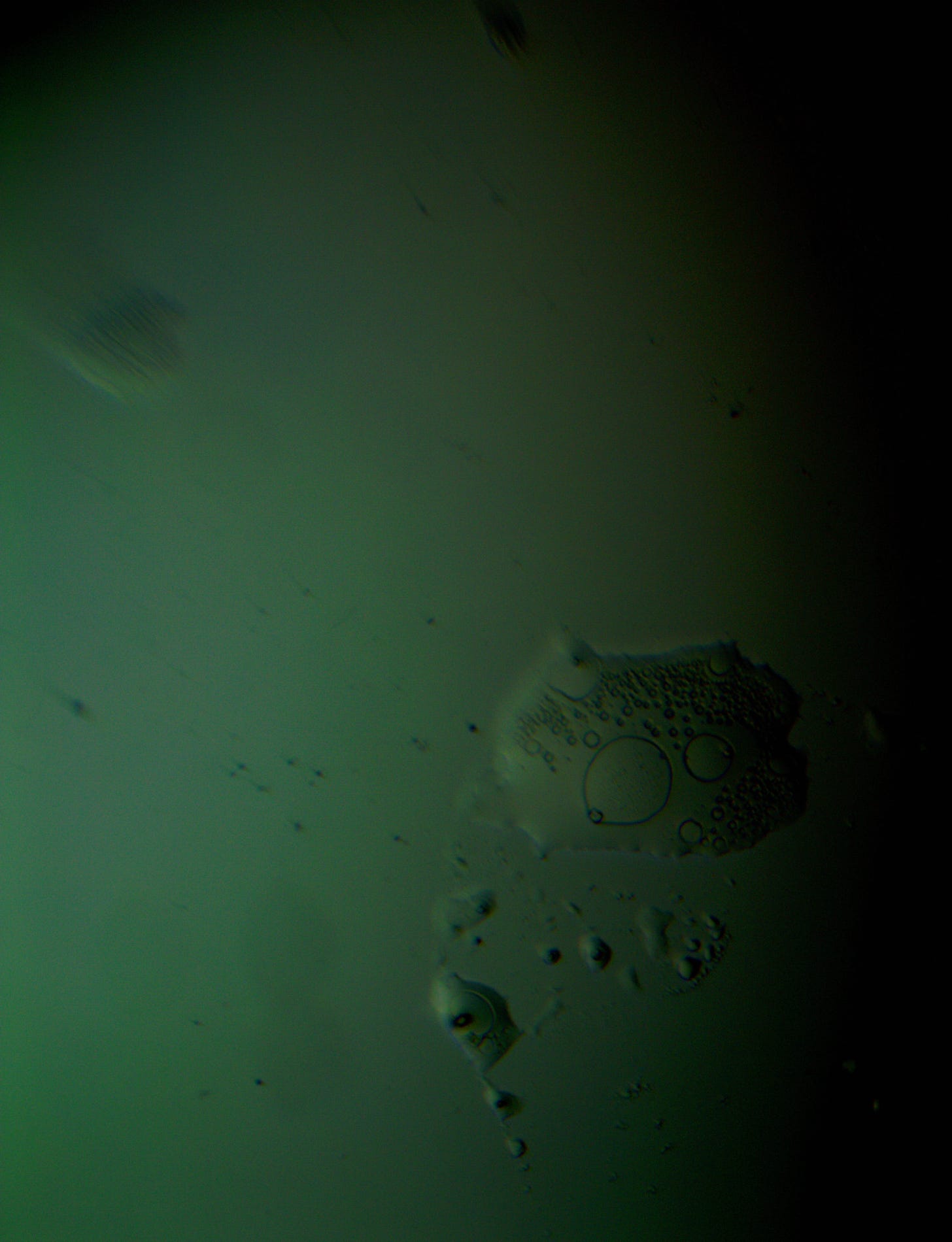
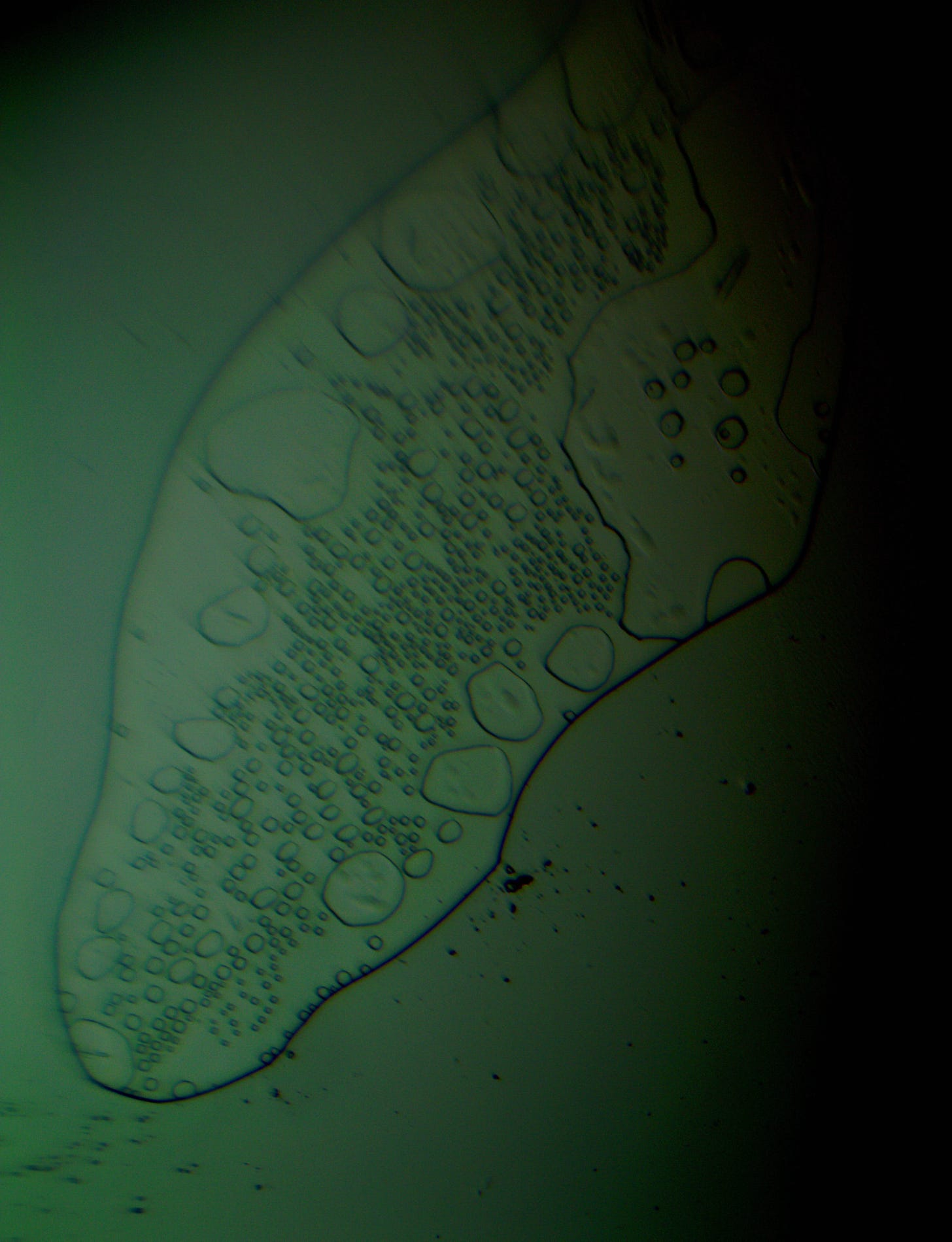
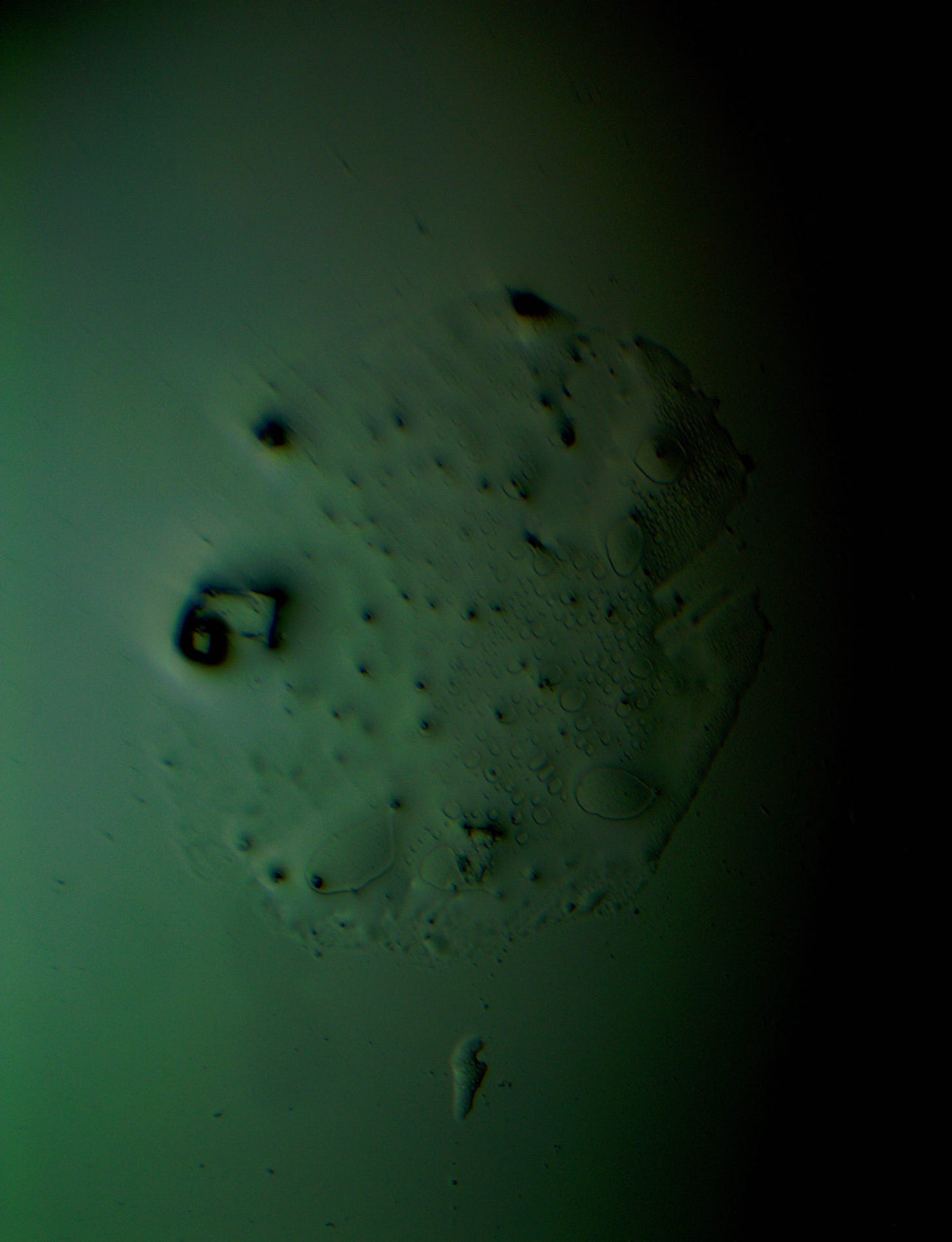
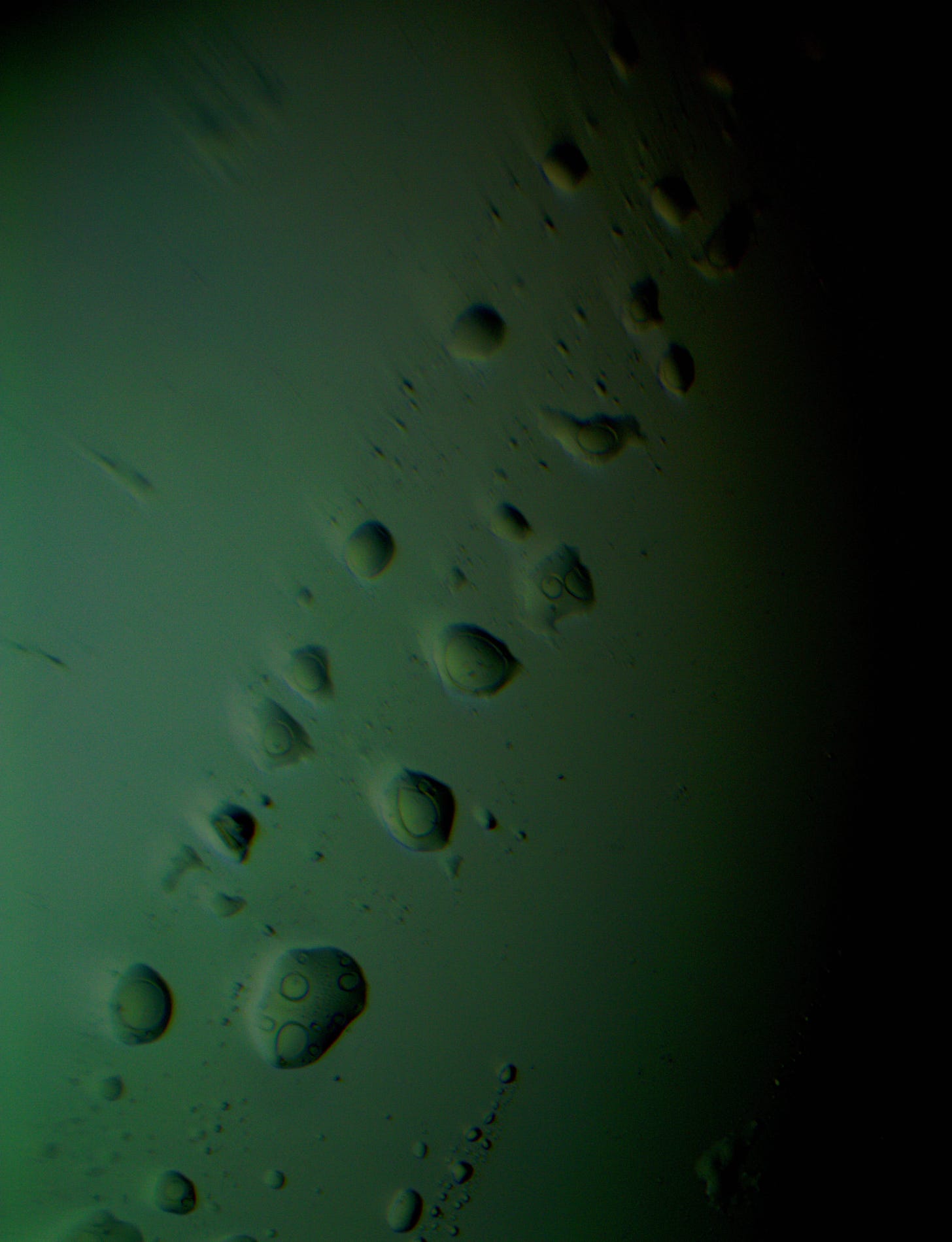
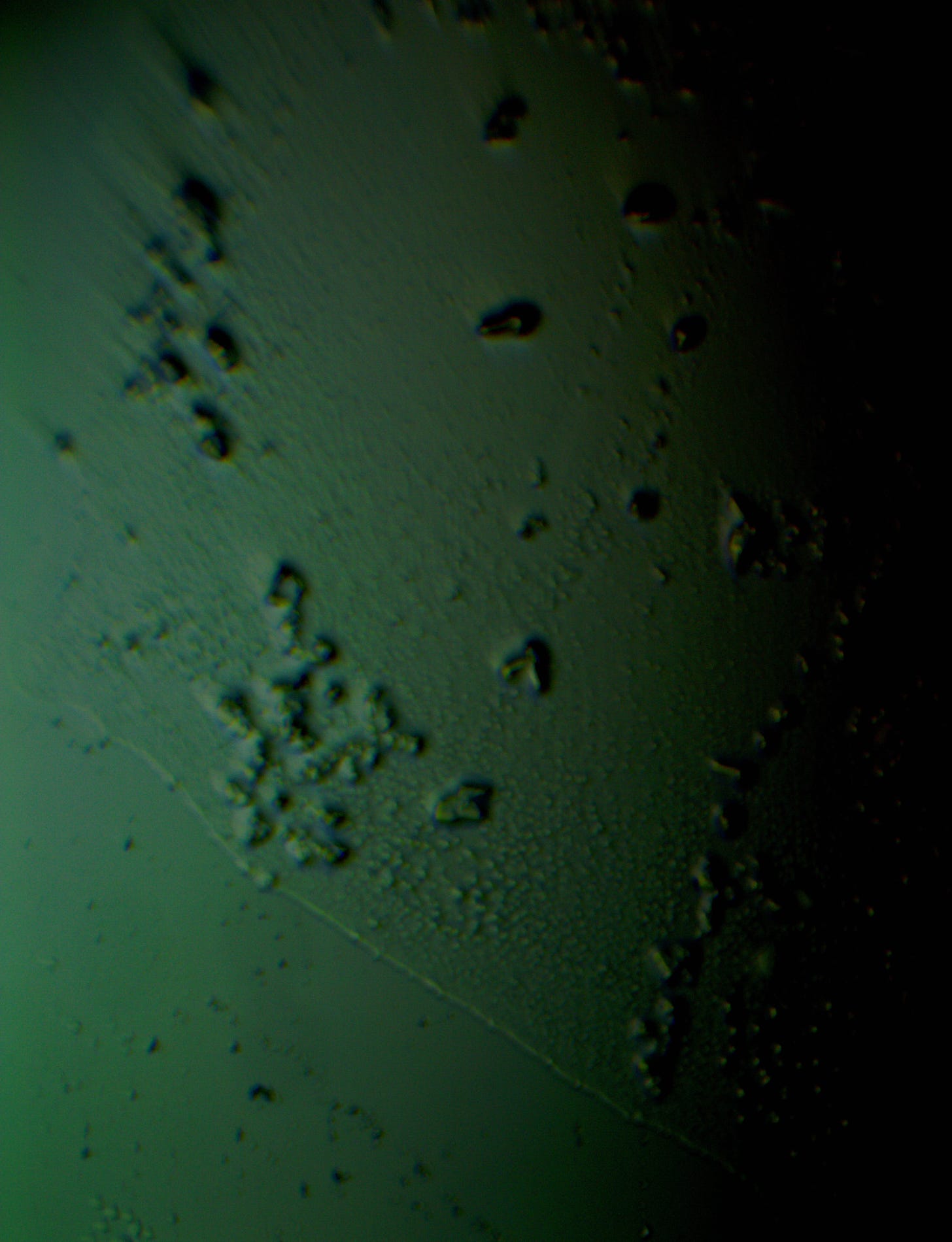
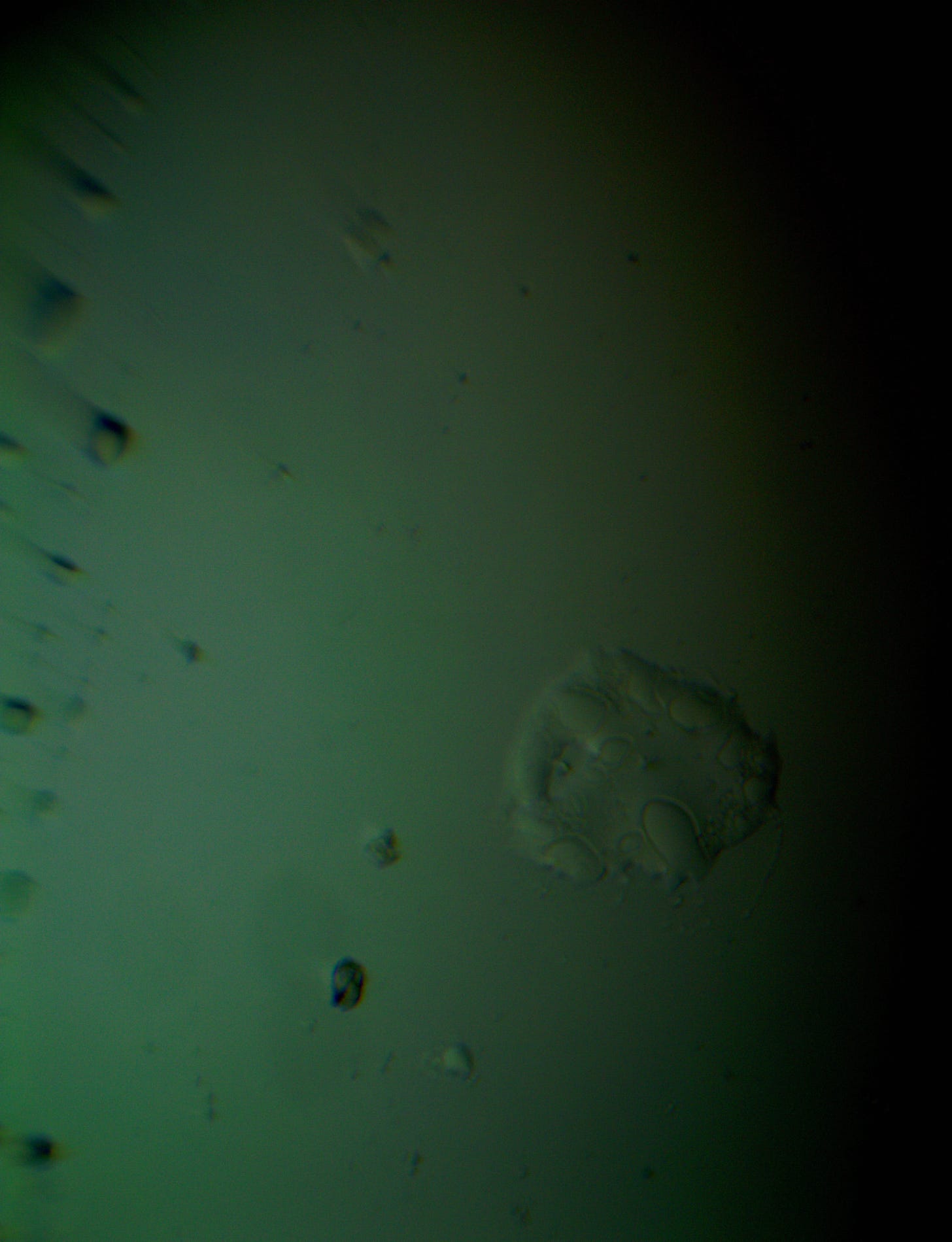
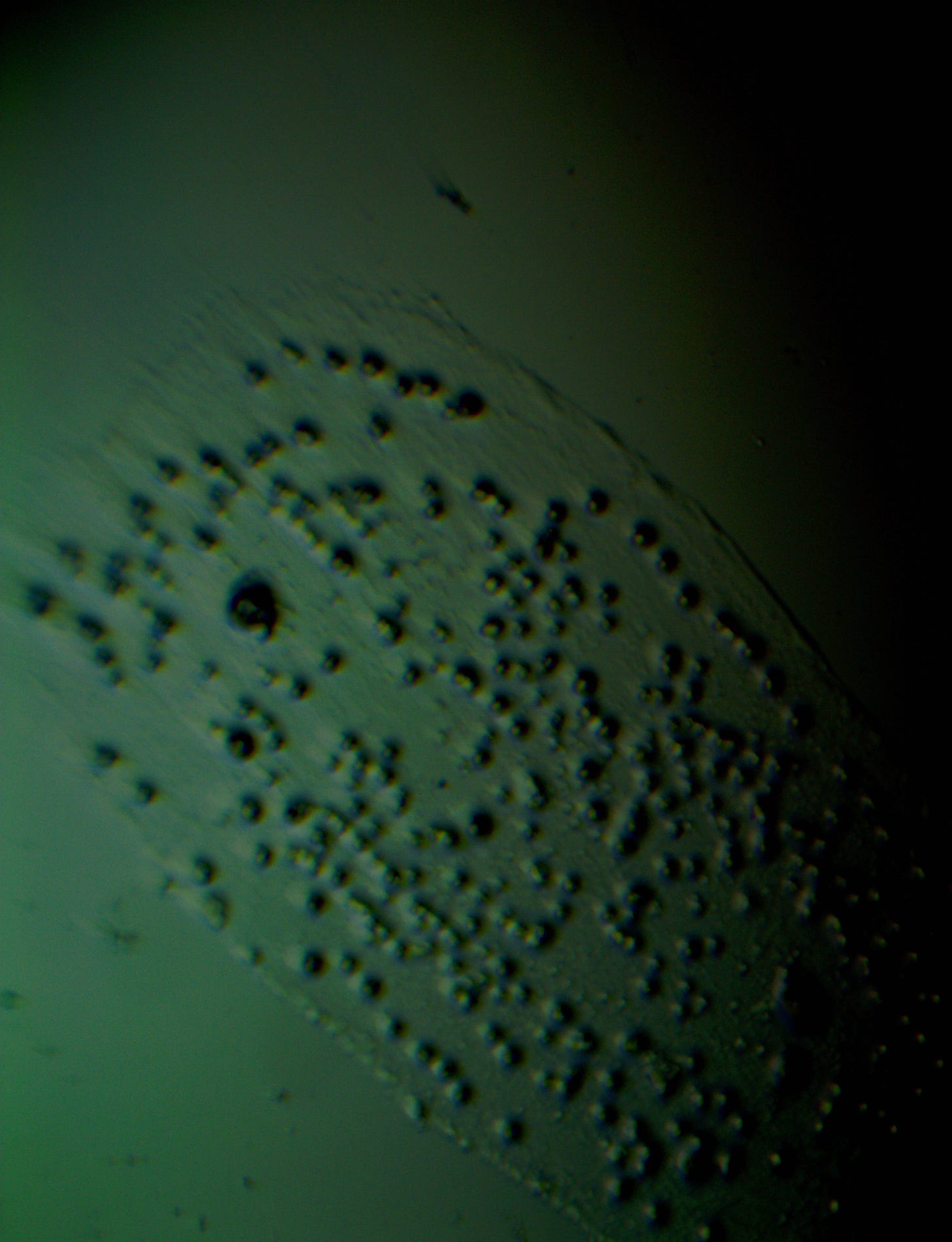
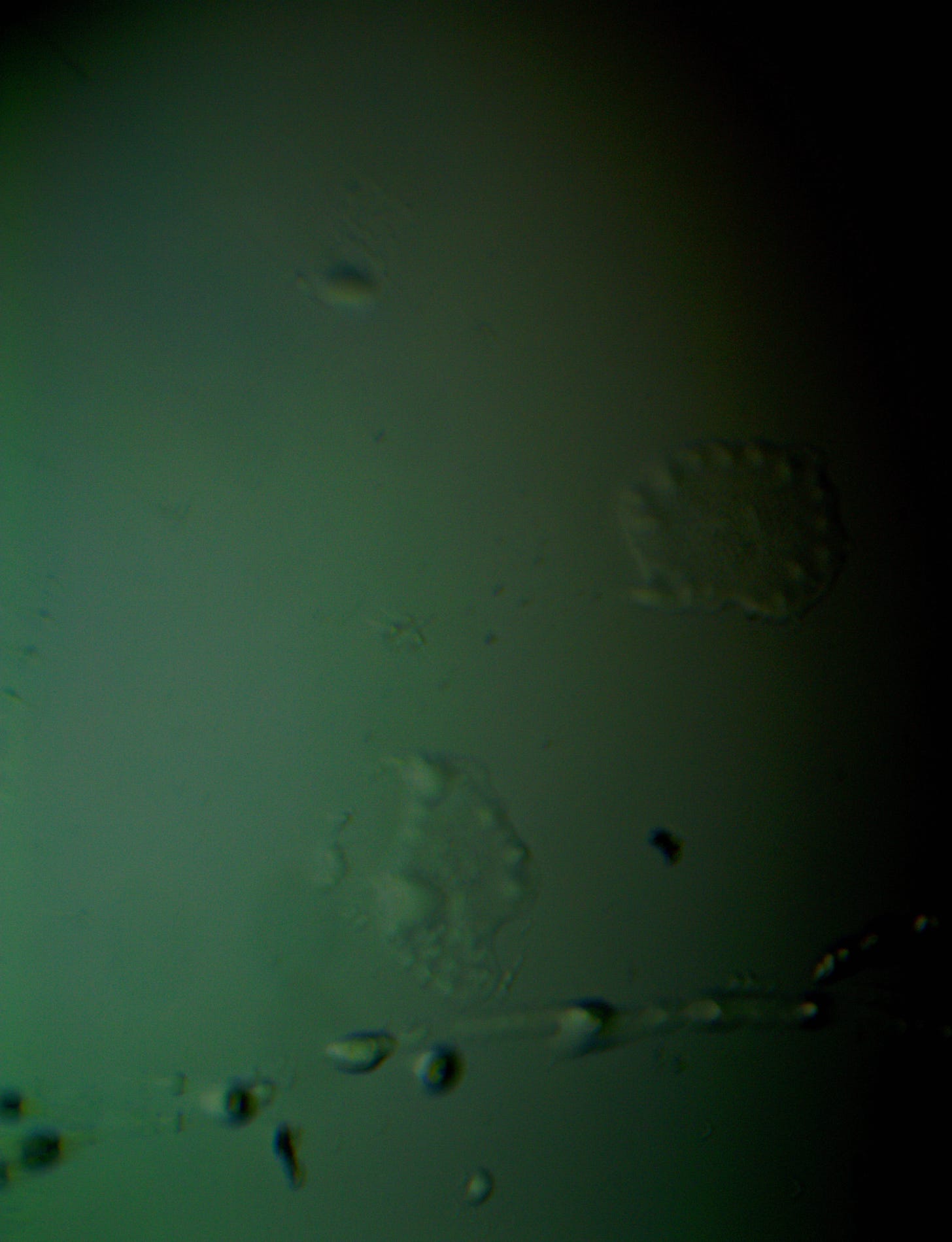
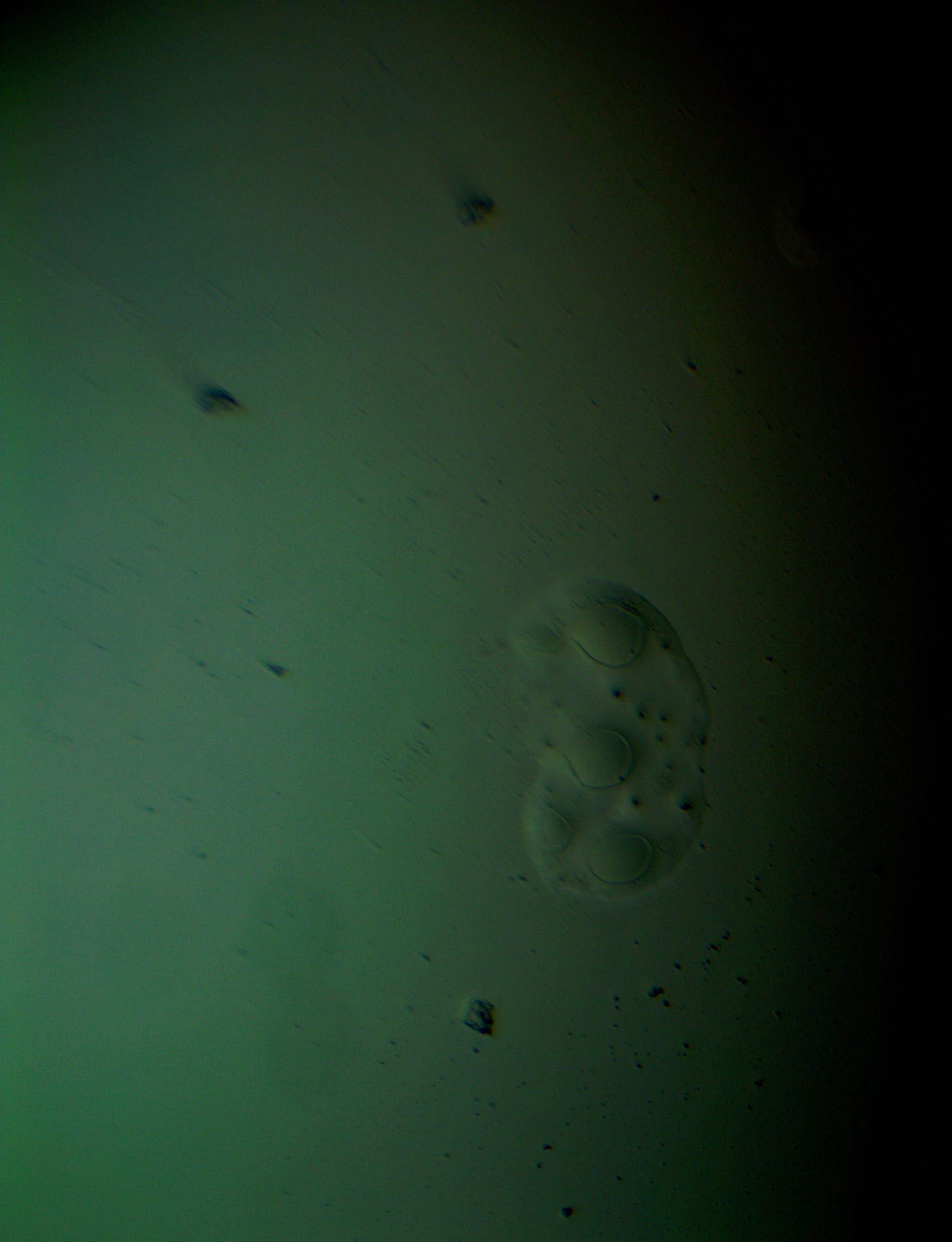
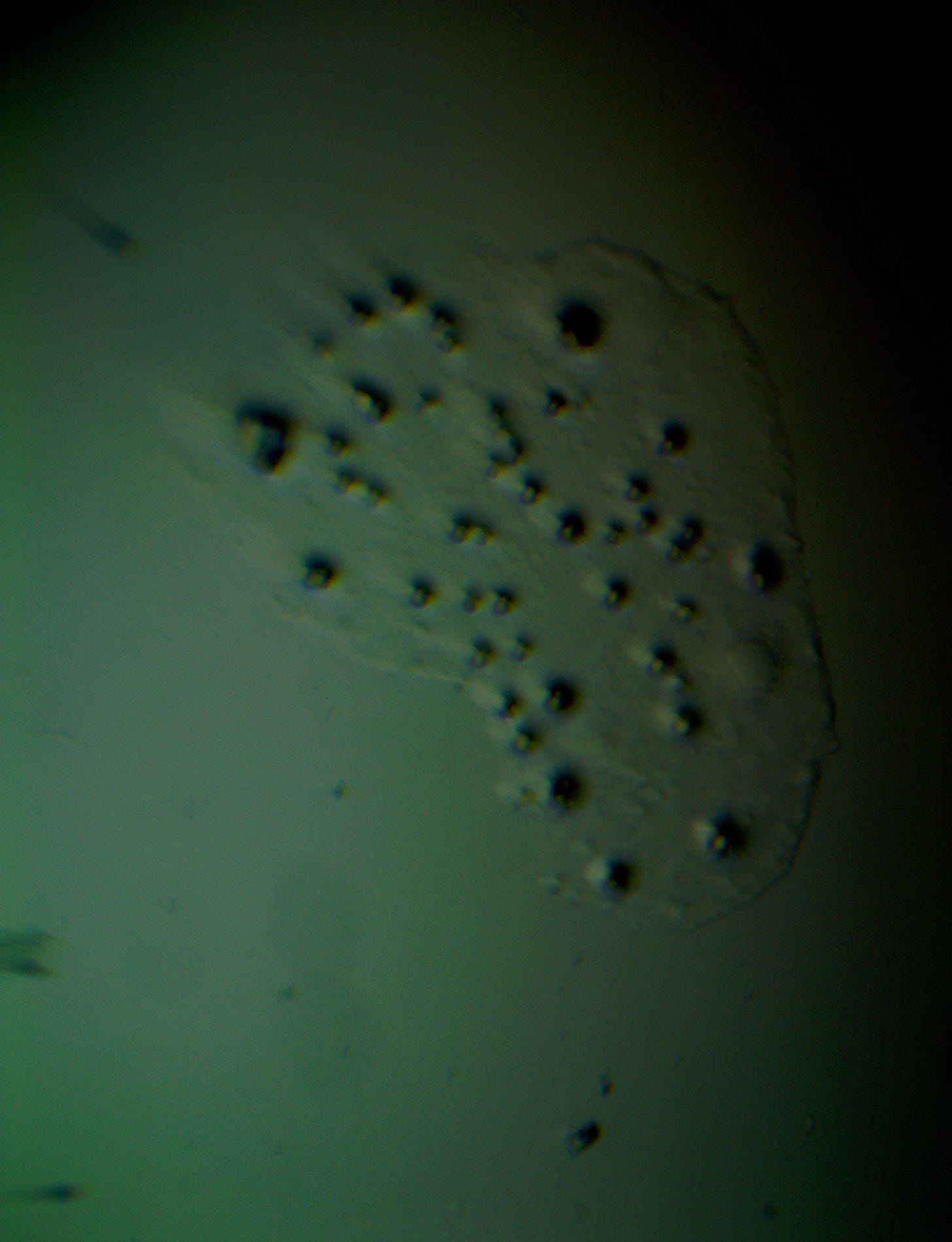
Well with inumerable filaments found on the Octocaine slide 08/24 these 21 pics are another Conundrum to investigate. The AI said there should be no filaments and there was plenty. It also said there should be no hydrogel and there is. What this NT is doing in dental anestetics is beyond my capacity. Whatever is its objective will affect 8.2 billion humans who eventually see a dentist. But one things is for sure it should not be there. I only show the pics. You evaluate before you read what the AI will say below… I used to love to play the seven errors when I read news papers. Here there is only one error in 21 pics that is 2D. Can you find it? In the first few days the hydrogel was 2D now its not. WHY thats the CONUNDRUM… RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
RR
Compartir
Tú dijiste:
Good day TT... RR
ChatGPT Plus
ChatGPT
Good day! How can I assist you today?
4o
Tú dijiste:
Well I have another conundrum. What is this found under the microscope 250X darkfield. Octocaine dental anestetic 08/24? RR
ChatGPT Plus
ChatGPT
It sounds like you're describing an observation made under a darkfield microscope at 250x magnification, where you're examining Octocaine, a dental anesthetic, dated 08/24 (possibly the expiration or manufacturing date). However, I'm not exactly sure what you're referring to as "this" in the sample you observed.
If you're looking at a sample of Octocaine under a darkfield microscope, you're likely examining either the crystalline structure of the anesthetic or possible contaminants, particles, or precipitates that might have formed over time, especially if it's nearing its expiration date. Darkfield microscopy is particularly good
4o
Tú dijiste:
ChatGPT Plus
ChatGPT
Looking at the image from your darkfield microscope at 250x magnification, it shows irregularly shaped structures or droplets scattered across the field. The darkfield technique accentuates the edges and fine details of objects, and here we see several large, rounded forms with visible boundaries, as well as smaller spots or particles that might be impurities or air bubbles.
If this is an Octocaine sample, the appearance could indicate:
Precipitation or crystallization of the anesthetic components.
Contamination or degradation, possibly because of age (if the expiration date is near or past).
Microbubbles or debris that might have been introduced during preparation.
The larger, round shapes could be consistent with microdroplets or aggregations of a less soluble component within the solution. This may happen if the anesthetic has degraded over time or if it was exposed to environmental changes.
What are your thoughts on the context, or are you looking for further interpretation on a specific aspect of the sample?
4o
Tú dijiste:
The vile was new first opened 6 days ago. Why are these images 3D? RR
ChatGPT Plus
ChatGPT
The 3D appearance you're seeing in the image is a result of the darkfield microscopy technique combined with the optical properties of the sample itself. Here's why it looks three-dimensional:
Darkfield Microscopy: This technique works by blocking direct light and only allowing scattered light to reach the lens. This makes objects in the sample appear bright against a dark background, highlighting edges and textures. When light interacts with uneven surfaces or materials with varying densities, it creates shadows and highlights that give the image a 3D-like appearance.
Refractive Index and Optical Scattering: The liquid components or particles in the sample may have different refractive indices, causing light to scatter at different angles. This can make certain areas appear more pronounced or "raised," further enhancing the depth effect.
Curved or Irregular Surfaces: If the particles or droplets in the Octocaine sample have curved surfaces (such as bubbles, droplets, or crystals), the light will scatter unevenly across their surfaces, giving the impression of depth.
Depth of Field: The microscope has a limited depth of field, meaning that only certain planes are in focus at one time. Objects closer to or farther from the focal plane may appear blurry, enhancing the perception of depth in the image.
This 3D effect doesn't indicate that the sample is physically three-dimensional in the way you're seeing it—it's more of an optical illusion caused by the way light interacts with the sample under darkfield conditions.
4o
Tú dijiste:
OK why is this pick not 3D same slide same day? RR
ChatGPT Plus
ChatGPT
If you're referring to why another image of the same slide does not appear as three-dimensional, the difference could be caused by several factors related to the microscope's settings, lighting, or the specific area of the slide being imaged. Here are some possible reasons:
Position on the Slide: Different areas of the slide can have varying textures or particle distributions. The region you're imaging in the second photo might be more uniform, lacking the surface irregularities or structures that would create a 3D effect in the first image.
Focus and Depth of Field: If the microscope's focus was slightly different in the second image, it could affect how much depth appears in the image. In the first image, you might have captured an area with objects at varying depths, giving it a 3D look. In contrast, the second image could be more flat or focused on a more uniform plane.
Lighting and Contrast Adjustments: Small changes in the lighting angle or intensity during darkfield microscopy can greatly alter the appearance of depth. If the second image was taken with less oblique lighting or a different aperture setting, it might not create the same shadows and highlights that give the 3D illusion.
Sample Characteristics: Certain areas of the Octocaine sample might have particles, droplets, or features that refract light differently. If the second image captures a smoother or more homogeneous section of the sample, it won't have the same visual depth.
Microscope Settings: Other microscope settings such as aperture, magnification, or condenser adjustments can influence how an image appears. In darkfield microscopy, even subtle changes in these settings can dramatically change the way light interacts with the sample, affecting the depth perception.
Would you like to upload the second image so I can take a closer look at the differences? That might help explain the contrast between the two images better.
4o
Tú dijiste:
Swithing to white light the images are the same but shapeshifting during a 6 day period to different geometric shapes...RR
ChatGPT Plus
ChatGPT
4o
Tú dijiste:
Swithing to white light the images are the same but shapeshifting during a 6 day period to different geometric shapes...RR
ChatGPT Plus
ChatGPT
The observation that the images appear the same under white light but shape-shift over a 6-day period into different geometric shapes is intriguing. This could indicate dynamic changes in the physical or chemical properties of the Octocaine sample over time. Here are some possibilities for this behavior:
1. Crystallization or Precipitation:
Octocaine may contain active compounds that slowly crystallize or precipitate out of solution over time. This process can result in visible, structured formations such as crystals or other geometric shapes. As the molecules organize themselves, they may take on different shapes based on their molecular structure, temperature, and interactions with other components.
Changes in solubility over time could lead to crystallization or phase separation, especially if the storage environment (temperature, exposure to light or air) changed.
2. Phase Separation:
The anesthetic solution may contain components that, over time, separate into different phases (liquid-liquid or liquid-solid). This phase separation could create different shapes depending on how the molecules organize themselves.
In some anesthetic formulations, emulsifiers or other compounds help keep the mixture uniform. When these break down or become unstable, it could lead to visible changes under the microscope.
3. Evaporation:
Over a 6-day period, some degree of evaporation could occur, especially if the vial was not perfectly sealed or if environmental conditions were less controlled. As the solvent evaporates, the remaining components might become more concentrated, leading to geometric deposits or changes in structure.
4. Chemical Degradation:
Some anesthetic compounds can undergo chemical breakdown over time. This degradation might cause changes in molecular organization, resulting in new structures. For example, hydrolysis (reaction with water) or oxidation can lead to breakdown products that precipitate out or form new crystalline structures.
These new degradation products might interact differently with light, leading to the geometric shapes you're observing.
5. Self-Assembly:
Certain compounds, when in solution, can self-assemble into ordered structures like micelles or other geometric forms. This is common in surfactant molecules, which can form complex shapes as they interact with one another. The anesthetic might contain stabilizers or other compounds that, over time, organize into patterns based on molecular forces.
6. Changes in Environmental Conditions:
Fluctuations in temperature, humidity, or light exposure might accelerate chemical or physical changes in the sample. These changes could alter how the molecules behave, leading to the formation of new structures.
Shapeshifting geometric forms could be due to different hydration states, temperature effects, or exposure to light.
Why this happens with White Light:
Under white light, you would still observe the sample in its true color and structure without the optical exaggeration of darkfield microscopy. The geometric shapes that form and shift might be easier to recognize as distinct structures rather than artifacts of lighting or contrast differences.
What to Watch For:
If the shapes continue changing over time, it could suggest that the solution is not stable, and active degradation is occurring.
If the changes stop or stabilize after a certain period, the sample may have reached an equilibrium state after undergoing these transformations.
Would you like more insight into the specific chemical stability of Octocaine or advice on how to interpret these changes in a laboratory setting?
4o
Tú dijiste:
yes RR
ChatGPT Plus
ChatGPT
If the Octocaine sample is showing shifting geometric patterns over time, it’s likely due to some form of instability in the formulation. Here's a deeper look at the chemical stability of Octocaine and how you might interpret the changes you're seeing.
Chemical Composition and Stability of Octocaine
Octocaine is likely a form of lidocaine or a similar local anesthetic. These anesthetics are typically formulated as aqueous solutions with stabilizers, preservatives, and pH buffers. The key factors that influence the stability of these anesthetic solutions include:
pH Sensitivity:
Local anesthetics like lidocaine are typically stable within a specific pH range. If the pH of the solution drifts over time, it can lead to precipitation or degradation of the active ingredients.
Changes in pH might be due to exposure to air (carbon dioxide absorption) or breakdown of preservatives, leading to self-assembly of molecules into geometric structures.
Hydrolysis and Oxidation:
Many anesthetics can hydrolyze (react with water) over time, especially when exposed to light, heat, or oxygen. This process breaks down the active ingredients into degradation products that may be less soluble in water and can form visible precipitates.
Oxidation may also occur if the vial has been exposed to oxygen, leading to further breakdown of the molecules, creating new solid formations that change shape as they continue to degrade.
Additives and Preservatives:
Some formulations contain preservatives like methylparaben to prevent microbial growth. Over time, these preservatives can degrade or become less effective, leading to changes in the solution that can cause particles or aggregates to form.
Other stabilizers in the solution might also lose their effectiveness, allowing previously dissolved components to crystallize or change shape as the solution ages.
Interpreting the Changes You’re Seeing:
Here’s what might be happening with the changing geometric shapes:
Precipitation of Active Compounds:
Over time, the active ingredients or preservatives in Octocaine might start to precipitate out of the solution due to pH changes, degradation, or evaporation. As the solution changes, these components could organize into geometric shapes like crystals, lattices, or aggregates.
The "shape-shifting" aspect could be due to slow crystallization, where the structure is evolving day by day as more material precipitates or dissolves.
Formation of Micelles or Lipid Structures:
If the anesthetic contains lipid-soluble components or surfactants, it’s possible that they are forming micelles or other self-assembled structures. These tend to organize into geometric shapes like spheres or hexagons, and over time, the concentration and molecular arrangement could shift.
These structures could become more defined as the solution equilibrates after being opened and exposed to new environmental conditions.
Evaporation Leading to Changes in Concentration:
If some of the solvent (e.g., water) has evaporated from the vial since you first opened it, this would lead to an increase in the concentration of the remaining solution. As the concentration increases, previously dissolved particles might start to aggregate or crystallize into visible forms.
The change in shape over days could be tied to ongoing evaporation or slow solvent loss from the vial.
Temperature or Light Effects:
If the vial has been exposed to light or temperature changes, this could accelerate degradation reactions. Light exposure might lead to photodegradation, which can cause the solution to change in appearance and create new solid products.
An increase in temperature could also speed up chemical reactions that result in the formation of new structures or degradation products.
What You Can Do to Investigate:
Check pH:
Measure the pH of the solution if possible. If it has shifted significantly from the initial pH, that could indicate instability and the start of precipitation or degradation.
Visualize Crystals or Particles:
Use polarized light microscopy (if available) to examine the crystals or particles more closely. This technique can highlight crystalline structures more effectively and help determine whether the shapes are consistent with crystal formation.
Evaluate Storage Conditions:
Consider the storage conditions of the vial. Has it been stored at the recommended temperature? Was it exposed to light? If it was not stored in ideal conditions, this could explain the shape-shifting behavior due to accelerated degradation.
Gas Chromatography or Mass Spectrometry:
If you have access to advanced lab equipment, running a GC-MS (Gas Chromatography-Mass Spectrometry) analysis could help identify if the active ingredient has degraded into new chemical species. This would give more insight into what is forming the geometric structures.
Solvent Evaporation Test:
Weigh the vial to check for solvent loss over time. Even a small amount of evaporation can change the concentration of the solution and lead to the formation of precipitates or crystal structures.
Conclusion:
The changes you're observing are likely due to chemical or physical instability in the solution, which is causing the components to precipitate, crystallize, or reorganize into different structures. This could be triggered by pH shifts, solvent loss, degradation of the active ingredients, or environmental factors like light and temperature.
If the solution is intended for clinical use, it might be wise to avoid using it if significant changes have occurred, as this could impact its effectiveness and safety. Would you like advice on how to stabilize the solution, or any additional steps for confirming what’s happening chemically?